

Calcium Chloride: CaCl2: 110.98 g/mol: Calcium hydride: CaH2: 42.094 g/mol: Calcium. Molecular weight of compounds : from Calcium chloride to Ethylpentane. Molar mass of 465 common chemical compounds Molecular. Incompatible with zinc, water, strong acids, methyl vinyl ether,bromine trifluoride, boron oxide, calcium oxide. Molar mass: 110.98 gmol 1 Appearance White hygroscopic powder Odor. Anhydrous calcium chloride Molecular Weight: 110. Table of data giving the molar mass of many common substances used in process industries. Appearance: white beads or powder OU Chemical Safety Data (No longer updated) More details.Experimental Density: 2.15 g/mL Alfa Aesar 44280Ģ.15 g/mL / 25 ☌ Kaye & Laby (No longer updated)ġ.71 g/mL / 25 ☌ Kaye & Laby (No longer updated).Soluble in ethanol Kaye & Laby (No longer updated) IUPAC Name: Calcium Chloride Other Names: Calcium(II) Chloride, Calcium dichloride Cas: 1 Chemical Formula: CaCl2 Molar Mass: 110.98 g/mol. The molar mass of calcium chloride - CaCl2 is:1 Ca + 2 Cl 40,078 2 x 35,45 110, 978 g. Soluble in acetone Kaye & Laby (No longer updated) Calcium Chloride Hexahydrate Exact Mass, 217.963684 Monoisotopic Mass, 217.963684 Signal Word, Warning Hazard Statements, H319 Hazard Codes, Xi. Experimental Solubility: 159% w/w in 100?C water Kaye & Laby (No longer updated)ħ4.5% w/w in 20?C water Kaye & Laby (No longer updated)įreely soluble in water and alcohol Alfa Aesar 12316.

Experimental Boiling Point: 1600 ☌ FooDB FDB015404ġ940 ☌ / 760 mmHg Kaye & Laby (No longer updated).Experimental Physico-chemical Properties.

Therefore number of moles are equal to 0.173 Mol. So for this question, its asking us, whats the actual molar concentration of the prepared solution So in order to do this, were going to first convert calcium chloride into moles. Therefore, number of moles will be equal to Mass is given which is 19.2g divided by the Molar Mass is 110.984 gram per mole. Where the molar mass of calcium chloride is 110.98 grams per mole. the ratio of number of atoms in Molecular weight calculation: 24 magnesium chloride. Supplier details: American Elements 10884 Weyburn Ave. Based on the atomic number, mass number, and neutron number of the. Relevant identified uses of the substance: Scientific research and development.
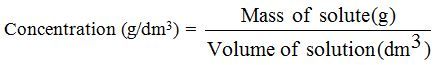
Therefore moller mass of calcium chloride is equal to 110 point nine years before unit is graham. Product Number: All applicable American Elements product codes, e.g. Calcium chloride dihydrate Certificates of Analysis. So 40.78 As there are two Atoms of Chlorine, so two into 35.453. Molar Mass Grade Value 1: 233-140-8: CaCl 2 HO: 147.01 g/mol: Ph Eur,BP,JP,USP: Pricing & Availability. So moller mass will be equal to there is one item of calcium. So first we will calculate the molar mass of calcium chloride. Where any number of moles, Ws mass and M. 77.5 grams This is Expert Verified Answer 47 people found it helpful ChoiSungHyun Explanation: Molar mass of CaCl2 110.98g/mol Moles of CaCl2 30.0 / 110.98 0. So the formula to calculate the number of moles is and is equal to w divided by M. (Molar mass of Ca 40.078 g/mol, Cl 35.453 g/mol, O 15.999 g/mol, Ag 107.868 g/mol, N 14.007 g/mol) A. Molecular Weight, 147.01, Computed by PubChem 2.1 (PubChem release 2021.05.07). Meanwhile, one carbon-12 atom has a mass of exactly 12 amu, i.e. We know that the mass of one mole of carbon-12 atoms is 12 g, or, 1 molm12C12 g, because of the definition of the mole. We now need to convert from amu to g/mol. Also the atomic weights that is molar masses of calcium and chlorine are given calcium has moller mass of 40.078 grandpa turmoil And Chlorine has moller mass of 35.453 g per mole. Calcium chloride CaCl2H4O4 - structure, chemical names. it shows us that calcium has a mass of around 40 amu. In the question, it is given that there are 19.2 g of calcium chloride And in 19.2 g we have to find out the number of moles, mm hmm.


 0 kommentar(er)
0 kommentar(er)
